ATLANTA, GA (August 21, 2023) — Emory University Professor Dennis Liotta has been featured in the Bayh-Dole Coalition’s new “Faces of American Innovation” report for developing chemical compounds used in life-saving medicines.
The Bayh-Dole Coalition is a diverse group of innovation-oriented organizations and individuals committed to celebrating and protecting the Bayh-Dole Act, as well as informing policymakers and the public of its many benefits.
On September 13, Dr. Liotta and four other leading innovators will receive the inaugural Bayh-Dole Coalition American Innovator Award in Washington, D.C.
“This is a wonderful honor, and I am humbled to receive it,” said Dr. Liotta. “Although I had no prior experience in drug discovery and development, at age forty I made the fateful decision to radically change the focus of my research and began what became a three-decade pursuit of the discovery of new drugs to address unmet medical needs. Against all odds, I was successful!”

Dennis Liotta, PhD / Photo: Stephen Nowland
Dr. Liotta discovered two chemical compounds capable of stopping HIV replication. [1] Emory later patented the molecules and licensed them to a drug company for further development. [2] By 2003, the FDA had approved both of Dr. Liotta’s novel compounds to treat HIV. Dr. Liotta’s lab also invented molnupiravir , which treats Covid-19. [3]
Dr. Deborah W. Bruner , Senior Vice President for Research at Emory University, commented, “Dr. Liotta has been at the forefront of bringing new research discoveries to market for over thirty years. His work bringing his discovery of Emtricitabine (in partnership with his two co-inventors) to market has provided a road map for other Emory researchers. He has blazed a trail for faculty and students to gain a deep understanding of pathways to engage with the entrepreneurial side of their research, and to fulfill our mission of doing good in the world by turning research into tangible benefits for humanity. As one of the top researchers of HIV antiretrovirals in the world, he is immensely deserving of this recognition.”
The Bayh-Dole Act was instrumental in bringing Dr. Liotta and his team’s technologies to the marketplace, said Joseph P. Allen, executive director of the Bayh-Dole Coalition.
“Without laws permitting universities to patent and license scientific discoveries made with the help of federal research funding, Dr. Liotta’s compounds may never have exited the lab and millions of people living with HIV/AIDS would not have healthy, productive lives today,” said Allen. “Thanks to the Bayh-Dole Act, private sector companies have a strong incentive to turn promising early-stage research into innovative therapies that save lives.”
“We’re thrilled for Dr. Liotta and this recognition,” said Todd Sherer, PhD, Associate Vice President for Research and Executive Director of Emory University Office of Technology Transfer. “Dennis’ work has directly impacted millions of lives, as most Americans who have HIV – and many around the world – take at least one of the antiretroviral drugs invented at Emory.”
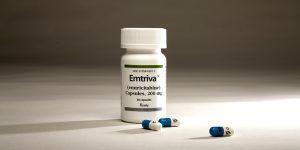
Emtriva, an HIV antiretroviral developed at Emory University by Drs. Dennis Liotta, Raymond Schinazi, and Woo-Baeg Choi.
Regarding his career, Dr. Liotta remarked, “Drug discovery is a team sport that requires synergistic interactions amongst multiple individuals with complementary skills. I have been fortunate to have many amazing co-workers and collaborators who have shared this journey with me. In particular, I want to acknowledge the important contributions of my friends and colleagues, Drs. Woo-Baeg Choi, Raymond Schinazi and George Painter.”
Emory signed a total of 10 exclusive and start-up patent licensing agreements in FY2022 alone, according to the Emory Office of Technology Transfer. [4] In the same year, the U.S. Patent and Trademark Office issued 45 patents to Emory inventors, including jointly held patents. [5] Over half of Emory-associated start-ups are working to discover new medicines. [6] More than 60 life-changing products, from novel diagnostics to virtual reality solutions, have reached the market so far. [7]
###

P: 404.221.0617
Fax: 404.448.3982
Email: admin@galifesciences.org
Address: 8607 Roberts Drive, Suite 250, Atlanta, GA 30350


